CBB042 Organic Chemistry - Reactions and Mechanisms
{`
Title of Access to HE Diploma: Access to Pharmacy and Biomedical Sciences
Unit title(s): Organic Chemistry - Reactions and Mechanisms
Unit code(s): CBB042
Level 3
`}
Scenario: You are a senior technician who has been asked to produce a PowerPoint on the synthesis of simple organic molecules, including mechanisms, creating questions and answers for trainee chemistry technicians. In your PowerPoint.
- Explain the benzene reaction and mechanism of benzene compound showing the steps of reaction to produce a final traction by using curly arrows. AC (1.1)
- Explain the differences between optical isomers and stereoisomerism using diagrams, to compare and contrast between them. AC (2.1, 2.2)
Complete the questions on the attached handout AC (1.1, 2.1, 2.2, 3.1, 3.2, 4.1)
Level 3 | Unit title: Organic Chemistry - Reactions and Mechanisms |
|
This assignment addresses the following Assessment Criteria from the unit (or a copy of the unit may be attached, if all AC are covered): | |
AC no | Level Three |
1.1 | Explain the reactions of benzene, including by reference to bonding and reaction mechanism(s). |
2.1 | Explain optical isomerism in organic molecules |
2.2 | Predict and explain the physical and chemical consequences of optical isomerism |
3.1 | Explain some of the different types of reaction mechanism in organic chemistry. |
3.2 | Construct reaction mechanisms. |
4.1 | Propose and justify viable pathways for the synthesis of organic molecules. |
Task 1 (AC 1.1)
- Explain why benzene sp2 hybridized. Use diagram.
- What types of bonding is present in benzene molecular? Use diagram
- What is delocalized chemical bond in benzene? Use diagram
Task 2
Benzene reacts with chlorine in the presence of a halogen carrier, such as AlCl3. (AC 1.1)
- Write the equation for the reaction of benzene with chlorine.
- How does the halogen carrier allow the reaction to take place?
- Outline a mechanism for this reaction. Include curly arrows and relevant dipoles.
- State the name of this mechanism.
- In contrast to benzene, the reaction of an alkene with bromine does not need a halogen carrier. Compare the different reactivities of benzene and alkenes towards chlorine.
Task 3
- How optical isomers arise in organic molecules. Use diagram (AC 2.1)
2-aminopropanoic acid (alanine) (AC 2.1)
This is typical of naturally-occurring amino acids.
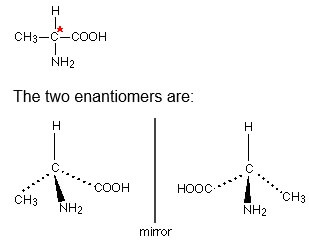
Only one of these isomers occurs naturally: the (+) form. You cannot tell just by looking at the structures which this is. Select and explain which one is it, left or right and why?
- Predict and explain the physical and chemical consequences of optical isomerism for the following structure: (AC 2.2)
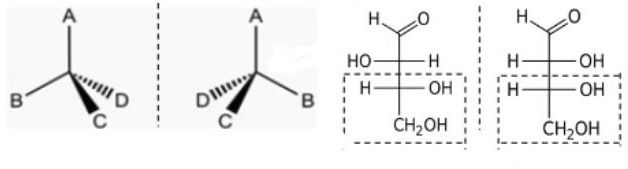
- Explain what is optical isomerism?
- Explain what is Diastereomers and Enantiomers on the above diagram.
- Explain the physical and chemical properties of above diagrams.
Task 4.
Explain and construct different types of reaction mechanism in organic chemistry
(AC 3.1, 3.2)
- Alkenes undergo electrophilic addition reactions to form saturated compounds.
- Define the term electrophile.
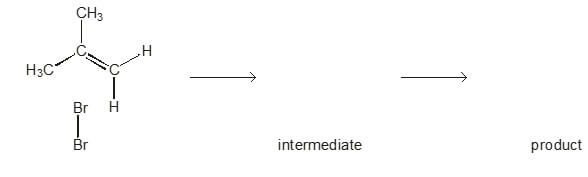
- The reaction between bromine and methylpropene is an electrophilic addition.
Describe, with the aid of curly arrows, the mechanism for this reaction. Show the intermediate and the product along with any relevant dipoles and lone pairs of electrons.
Propanal, CH3CH2CHO, can be used in the synthesis of organic compounds.
CH3CH2CHO reacts with NaBH4 in a nucleophilic addition reaction. The nucleophile can be represented as a hydride ion, H–. A mechanism for the reaction is shown below.
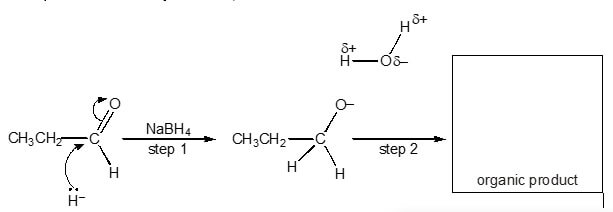
- Add curly arrows to the mechanism to show how the intermediate reacts with the water molecule in step 2.
- Draw the structure of the organic product in the box above.
- What is meant by the term nucleophile?
- Isomer L, C5H10, reacts with CI2 in the presence of UV light to produce the organic product C5HgCI. The reaction takes place in three stages: initiation, propagation and termination.
- The reaction is initiated by the fission of CI2. State the type of fission involved.
- Write an equation to illustrate the fission of CI2 in (i).
- The fission of CI2 leads to a chain reaction involving two propagation steps. Complete the equations for the two propagation steps.
C5H10 ............ C5H9 .............
C5H9 ............ ........ .............
Task 5
In the laboratory, ethanol can be oxidised with acidified potassium dichromate(VI). (AC 4.1)
- The ethanol can be oxidised to form either ethanal, CH3CHO (Fig. 1), or ethanoic acid, CH3COOH (Fig. 2).
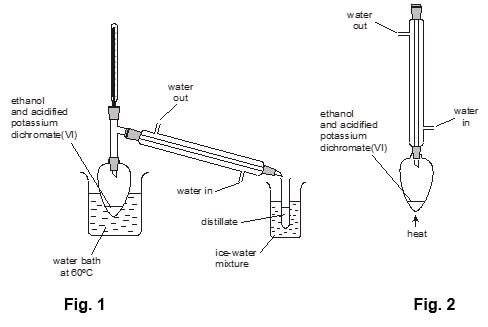
The boiling points of ethanol, ethanal and ethanoic acid are given in the table below
CH3CH2OH
CH3CHO
CH3COOH
boiling point / °C
8
21
118
Use this table of boiling points to explain:
- why the organic product is likely to be ethanal if the apparatus shown in Fig. 1 is used,
- why the organic product is likely to be ethanoic acid if the apparatus shown in Fig. 2 is used.
- Write a balanced equation for the oxidation of ethanol to ethanoic acid. Use [O] to represent the oxidising agent.
- identifying a series of reactions to give a desired synthetic pathway:
- Starting from Alkane ending with Ester, stating the materials and conditions plus chemical equations.
- To demonstrate an understanding of the characteristic tests which distinguish organic molecules, you will need to carry out a series of practical tests to identify the functional groups of unknown chemicals, followed by a full written report
Task 6
Explain with diagrams the selectivity and stereospecificity in organic reactions. (AC 4.1)
Homework Help UK
- HND Assignment Help
- Do my assignment UK
- IMarEST Ceng report for the UK council
- Business Plan Writing for the new UK Innovator VISA
Sample Assignments UK
- Individual Project Development
- CORC373 Advanced Object Oriented Programming
- CSY3024: Databases 3
- CBB042 Organic Chemistry
- ENG490 - Introduction to Petroleum Engineering
- COMP1664 Network Technology
- IB9EN0 Financial Markets
- IMC Campaign Planning Project
- BM019 Operational Supply Chain Management
- IB98W0 Management Accounting And Control
- BTEC Pearson Higher Nationals in Computing Unit 01
- BTEC Pearson Higher National Diploma in Computing Unit 11
- BTEC HND in Computing Unit 6 Managing a Successful Computing Project
- Business Plan on Cafe in UK
- Summarise monzo current business model
- A global challenge that is experienced by a company
- MOD004051 Finance for Decision Making
- UK BTEC HND in Computing ECE2072 ASSIGNMENT
- UK BTEC HND in Computing Unit 15: Transport Network Design
- UK BTEC HND in Computing Unit 16 Cloud Computing
- UK BTEC HND in Computing Unit 30 Application Development
UK Universities
- Abertay University
- Aberystwyth University
- Bangor University
- Birmingham City University
- Coventry University
- Huddersfield University
- Lancaster University
- Leeds University
- Leicester essay writing help
- Loughborough University
- Kingston University
- Manchester Essay Writing Help
- Manchester Metropolitan University
- Middlesex University
- Newcastle University
- Northumbria University
- Nottingham Trent university
- Oxford Brookes University
- Regents University
- Robert Gordon University
- Royal Holloway
- University of Arts London
- University of Bedfordshire
- University of Birmingham
- University of Bradford
- University of Bristol
- University of Dundee
- University of East Anglia
- University of East London
- University of Glasgow
- University of Greenwich
- University of Hertfordshire
- University of Kent
- University of Law
- University of Lincoln
- University of Manchester
- University of Nottingham
- University of Porstmouth
- University of Reading
- University of Sheffield
- University of Southampton
- University of St. Andrews
- University of Stirling
- University of Strathclyde
- University of Surrey
- University of Ulster
- University of Warwick
- University of Westminster
- University of Winchester
Testimonials
The assignment I got was complicated enough and it was hard for someone else to do it. Our professor had explained a proper technique and format to do it, I was worried if urgenthomework could do it or not? I had a talk with their customer care and they gave me the contact details of the expert who would do my work. I told them the procedure and I was surprised by the product delivery. It was an excellent work framed in the style and Format I wanted it.
I had a critical task accommodation due date. One of my companions recommended that I should hire the services of urgenthomework.com. When I put in the task request, they quickly acknowledged it and comprehended the earnestness and urgency of time. they conveyed my task until before a day of submission date. Services are as good as your writing is. And quite affordable as well. Wish you good luck for the future. Keep growing.
I have been using this website since last many years. It helps me in my college project and homework. Excellent study materials are provided which is easy to understand and learn. Read More

