Enzyme Linked to Immunosorbent Assay
Enzyme-linked to immunosorbent assay (ELISA)
Introduction and Aim
ELISA is a plate-based test technique for detecting and measuring soluble molecules such as peptides, proteins, antibodies, and hormones. The same method is also known by other names, such as enzyme immunoassay (EIA) (Li et al.2019). Enzyme-linked immunoassay (ELISA) is an acronym for enzyme-linked immunoassay. Antibodies in the blood are detected using this routine laboratory test. When the body's immune system identifies dangerous molecules called antigens, it produces an antibody (Mirhosseini et al.2017). The enzyme-linked immunosorbent assay (ELISA) is a method for detecting the presence of antigens in biological materials. Marion M. Bradford invented the Bradford protein test in 1976. It is a spectroscopic analytical approach for measuring the concentration of protein in a solution that is quick and accurate (Mohammed et al.2021). The amino acid makeup of the tested proteins affects the response. The Bradford protein test is used to determine the total protein concentration in a sample. The assay works on the idea that when protein molecules attach to Coomassie dye in acidic circumstances, the colour changes from brown to blue (Mohammed et al.2021). The Bradford assay is quick and requires roughly the same quantity of protein as the Lowry assay. It is fairly accurate and can detect out-of-range samples.
The aim of this experiment is to isolate the antigens of E.coli and S.typhi followed by the determination of the protein concentration present in them. Then the secondary aim of this experiment is to find out the volume of protein needed to coat the ELISA plate and finally running of indirect ELISA to detect the specific antigens present in both the sample microbes.
Materials and Methods
The materials needed for the above stated experiments are ELISA plates, Bradford reagent, Coomassie blue, high frequency sound wave emitter, sample bacteria – E. coli and S.typhi, centrifuge and UV -Vis spectrophotometer.
The antigen from the two microorganisms were isolated following their determination of protein concentration by Bradford method was carried out. Then the volume of protein required to coat the ELISA plate was measured. Finally, indirect ELISA was done to determine specific antigen presence in the microorganisms. The observations were recorded as given in the results.
Results
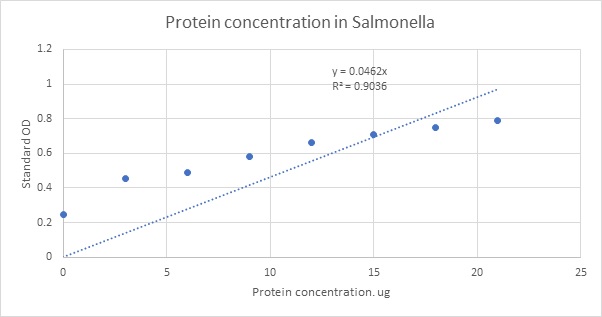
Fig 1: The protein concentration of Salmonella has been shown in the X axis and as it increases the standard optical density, or the absorbance also increases due to increase in turbidity of the culture solution.
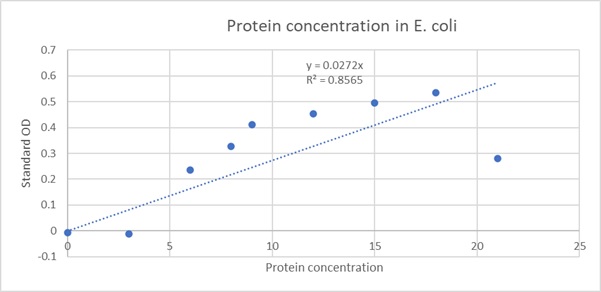
Fig 2: The protein concentration of E. coli has been shown in the X axis and as it increases the standard optical density, or the absorbance also increases due to increase in turbidity of the culture solution.
Final concentration in the sample

The protein volume needed for ELISA plate coating for E.coli
Concentration of protein in E.coli sample:
y = 0.0272x
Therefore, x = 1.028 µg/µL
C1 = 1.028
V1 = ?
C2 = 0.005
V2 = 3000
Thus,
V1 = (0.005 x 3000)/1.028 = 14.59 µL
The protein volume needed for ELISA plate coating for S.typhi –
Concentration of the protein in Salmonella sample:
y = 0.0462x
Thus, x = 0.556 microgram/microliter
Therefore,
C1 = 0.556 µg/µL
V1 = ?
C2 = 0.005 µg/µL
V2 = 3000 µL
V1 = (0.005 x 3000)/0.556 = 26.98 µL
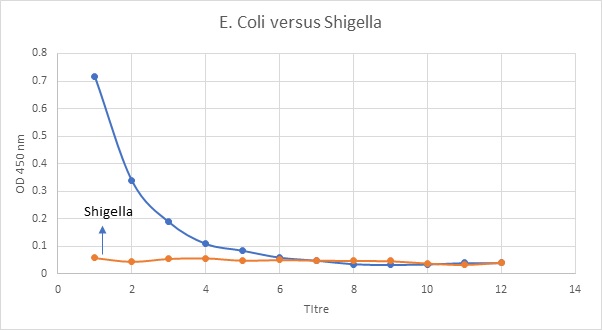
Fig 3: ELISA detection of E. coli using anti-Salmonella H-antigen antibody and anti-Shigella antibody (Negative control)
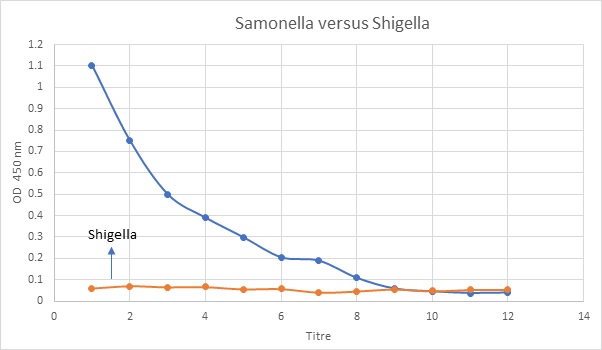
Fig 4: ELISA Detection of Salmonella Typhymurium 82/695 H-antigen using anti Salmonella H-antigen antibody and Shigella antibody (negative control)
Discussion
The titre value for E.coli was lesser than Salmonella. This means that when the patient has a concentration of 7 microgram/microliter E.coli., he or she can be stated to be infected whereas for Salmonella, the value is 9. This means higher concentration of Salmonella antigen is needed for infection when both the test microorganisms are compared to Shigella. Also, these results are satisfied by the results obtained from the first part of this experiment where the protein concentrations were measured. It was found that E.coli antigen concentration was lesser in the patient than the Salmonella. The results of this experiment were same as expected since it has been found that the ELISA was also successful since the titre values were shown by the intersecting points. Since, E.coli has been found to be the normal inhabitant microflora of the human body, the overall concentration should be expected to be higher. However, since the same was found to be lower, it can be said that the patient was affected by Salmonella in which required a higher threshold concentration when compared with E.coli on measuring against Shigella.
References
Dehghani, B., & Rasooli, I. Sensivity and specificity of Serum Antibody against InvH recombinant protein of Salmonella Paratyphi A, B and C.
Li, Q., Zhu, Y., Yin, K., Xu, L., Yin, C., Li, Y., ... & Jiao, X. (2019). Purification of recombinant IpaJ to develop an indirect ELISA-based method for detecting Salmonella enterica serovar Pullorum infections in chickens. BMC veterinary research, 15(1), 1-7.
Mirhosseini, S. A., Fooladi, A. A. I., Amani, J., & Sedighian, H. (2017). Production of recombinant flagellin to develop ELISA-based detection of Salmonella Enteritidis. brazilian journal of microbiology, 48, 774-781.
Mohammed, G. M., ElZorkany, H. E., Farroh, K. Y., Abd El-Aziz, W. R., & Elshoky, H. A. (2021). Potential improvement of the immune response of chickens against E. coli vaccine by using two forms of chitosan nanoparticles. International Journal of Biological Macromolecules, 167, 395-404.
Wang, C., Xing, K., Zhang, G., Yuan, M., Xu, S., Liu, D., ... & Lai, W. H. (2019). Novel ELISA based on fluorescent quenching of DNA-stabilized silver nanoclusters for detecting E. coli O157: H7. Food chemistry, 281, 91-96.
Yin, K., Ren, J., Zhu, Y., Xu, L., Yin, C., Li, Y., ... & Jiao, X. (2019). Application of monoclonal antibodies developed against the IpaJ protein for detection of chickens infected with Salmonella enterica serovar Pullorum using competitive ELISA. Frontiers in veterinary science, 6, 386.
Resources
- 24 x 7 Availability.
- Trained and Certified Experts.
- Deadline Guaranteed.
- Plagiarism Free.
- Privacy Guaranteed.
- Free download.
- Online help for all project.
- Homework Help Services

